Suk Hoon Kang, Ki Suk Jang,
, Macromolecules 2007, 40, 8349-8354.
DOI:10.1021/ma0712293
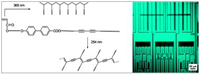
Fig.: A series of heterobifunctional mesogenic biphenyl esters having two different polymerizable groups, i.e., acryl and diacetylene groups, were synthesized and their thermal behaviors and polymerization investigated. All compounds showed enantiotropic transitions. Under POM, highly birefringent focal-conic fan textures appeared on heating and cooling from the isotropic melt. Compounds 6-8 having a butyl spacer between a biphenyl and a diacetylene group exhibited LC phases even at room temperature. The X-ray diffractograms of compounds 6-8 showed a set of reflections in the small-angle region. They consisted of more than three sharp diffraction peaks with d spacings in the ratio of 1:1/2:1/3, showing that the compounds had well-defined smectic A structures. For the photoimaging a mixture of 6 and a photoinitiator (2,2-dimethoxy-2-phenylacetophenone, 4 wt%) was cast on a glass plate and sheared with a cover glass at room temperature to result in an LC monodomain. The acryl group was then selectively polymerized by irradiation with low-intensity 365 nm UV light to yield a polymer film. Subsequent UV irradiation at 254 nm using a 100 W high-pressure mercury arc lamp through a photomask produced conjugated polyacetylene chains in the irradiated area. The polydiacetylene chains were fluorescent, and the patterned image was directly visualized by fluorescence microscopy.